Our research is focused on three main areas (see links below) The first section is a general introduction.
How does cytoskeletal force generation drive cell movement?
Which molecular mechanisms underlie the mechanics of retraction?
How is the movement of epithelial cell sheets coordinated during the wound healing process?
How does cytoskeletal force generation drive cell movement?
Cell movement is a physical process whereby protrusion and adhesion formation occur at the front, while contractile forces facilitate cell detachment and retraction at the rear. Each of these processes depends on force generation by the actin cytoskeleton. The polymerization of actin filaments at the front pushes out the cell edge, while the interaction of actin and myosin II generate contractile forces that tend to pull the cell edge inward. The formation of new adhesions at the front edge provides an adhesive force that anchors it to the substrate. Retraction at the rear edge depends on reducing adhesive forces and increasing contractile forces in this region so that it can detach. Although much is known about the molecular basis of these individual processes, it is not clear how they are coordinated with one another to produce movement.
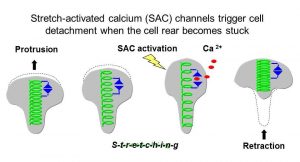
We are using the fish epithelial (skin) keratocytes to address this question because they can move rapidly in one direction while maintaining a simple “fan” shape. We have previously shown that this rapid, efficient mode of movement depends in part on the activation of stretch-activated calcium channels (SACs, Lee et al., 1999).
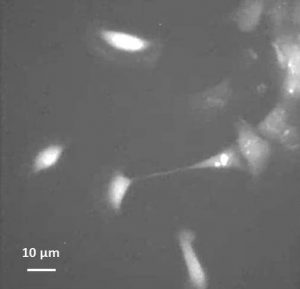
A group of moving keratocytes loaded with calcium indicator (click for movie). Note the cell that is stretched and attempting to break away from the edge of a sheet of cells. Transient increases in [Ca2+]i are shown as brief increases in fluorescence, which are more frequent in this stretched cell than following detachment at the rear. Movie duration is 10 minutes.
When the cell rear becomes stuck, SACs trigger a transient increase in intracellular calcium [Ca2+]i which leads to retraction, allowing movement to continue. Thus by acting as mechanosensors SACs coordinate protrusion with retraction, which promotes rapid motility. The work of others has shown that cell adhesions can also act as mechanosensors. The major goal of our work is to understand how mechanosensing by SACs and adhesions work together to coordinate different cytoskeletal functions (see below).
Which molecular mechanisms underlie the mechanics of retraction?
Cell movement consists of repeated rounds of protrusion at the front and retraction at the rear. While much research has focused on the molecular mechanisms and biophysics of protrusion, less attention has been paid to the mechanisms of detachment at the rear, and yet retraction is often the limiting step in the cycle of cell motility. This is particularly apparent in cells, like fibroblasts, which form large stable focal adhesions at the front and rear of the cell. The current dogma is that myosin II dependent contractile forces are involved directly in peeling up the rear edge. However, in fast moving cell types such as keratocytes (Morin et al., 2014) and Dictyostelium amoebae (Lombardi, et al., 2007) we have found evidence for other mechanisms of detachment that are less dependent on contractile forces or can occur independently of them. This suggests that the molecular mechanisms of detachment in fast moving cell types may differ from slow moving ones. The overall goal of this work is to relate molecular mechanisms to the mechanics of retraction, in particular how SAC mediated calcium transients induce retraction.
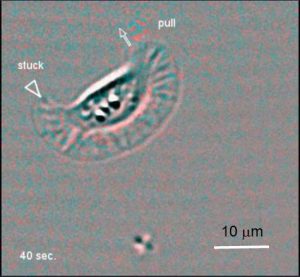
A keratocyte displaying a "recoil" and "pull" type retraction
A series of difference* images obtained from a keratocyte moving on an elastic surface embedded with marker beads. This cell exhibits a "recoil" type reaction on its right side, followed by a "pull" type retraction on its left (more). The flash of red speckles close to a retracting edge is caused by cell detachment that allows the displaced marker beads to return to their original positions. *Difference images are obtained by subtracting each image in a sequence from the next. These images highlight changes that occur between image frames.
We have previously shown that SAC mediated calcium transients trigger retraction when the cell rear becomes stuck (Lee et al., 1999) and that this involves the local regulation of adhesion strength (Doyle and Lee, 2005). To address this question, we have used traction force microscopy (TFM) to measure the local changes in traction stress at the rear of the cell that occur during retraction, and have identified four distinct modes of detachment (Morin, et al., 2014). To investigate which signaling pathways are involved in retraction we treated keratocytes with inhibitors that blocked or augmented both calcium dependent and independent regulation of contractility (Morin et al., 2014) while recording the effects on the mechanics of detachment using TFM. We found that “recoil” retractions, often followed a SAC mediated calcium transient and involves the activation of myosin light chain kinase (MLCK). We propose that this calcium dependent enzyme triggers detachment by locally increasing the turnover rate of adhesions in a force dependent manner.
One approach that we are using to test this possibility, is to monitor the turnover rate of adhesions using fluorescence recovery after photobleaching (FRAP) during calcium induced increases in contractility, following the photo-release of a caged ionophore. If we can confirm that calcium dependent, force-induced adhesion disassembly occurs in keratocytes this will suggest that fast moving cell types do not “pull up” the rear edge but “release” it instead. Such a finding would imply that other fast moving cell types may employ a fundamentally different mode of detachment than slow moving cell types in vitro.
A typical "recoil" retraction
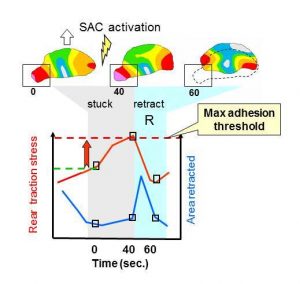 To characterize the mechanics of retraction, a series of traction magnitude maps were obtained from a moving keratocyte (open block arrow) whose left rear edge becomes temporarily stuck (see boxed area at 40 sec.) This triggers the activation of stretch-activated calcium (SAC) channels and leads to an increase in local traction stress, followed by an abrupt retraction (R, see animation).
To characterize the mechanics of retraction, a series of traction magnitude maps were obtained from a moving keratocyte (open block arrow) whose left rear edge becomes temporarily stuck (see boxed area at 40 sec.) This triggers the activation of stretch-activated calcium (SAC) channels and leads to an increase in local traction stress, followed by an abrupt retraction (R, see animation).
Measurements of local traction stress (TSl, red line) were taken at the indicated times in the boxed region during retraction R (blue region on graph, dotted outline on traction map). Comparison with the area retracted (blue line) shows that when the rear becomes stuck (grey region) traction stress increases while the area retracted is negligible. When traction stress exceeds the adhesion threshold traction stress drops, while the area retracted increases during retraction.
The use of zebrafish transgenics to study the role of mechanosensing in the regulation of cell movement
To study the molecular dynamics of the cytoskeleton and associated adhesion sites involves the expression of various fluorescently tagged proteins. However, since keratocytes are a non dividing cell type, it is difficult to transfect them with the proteins of interest or to generate cell lines expressing these proteins. The genetic manipulability of the zebrafish offers a solution to this problem. Techniques for generating transgenic zebrafish embryos are well-established (Liu and Leach, 2011) including methods for driving keratocyte specific protein expression (Raymond, et al., 2014) and isolating keratocytes from early embryos (Lou, et al., 2015).
The goal of our current work is to generate transgenic zebrafish embryos whose keratocytes express specific fluorescently tagged adhesion proteins so that we can measure local changes in adhesion dynamics in relation to [Ca2+]i and cytoskeletal contractile force (more). Adopting the zebrafish as a model system could open up new fields of study, for example, the use of force biosensors to study the collective movement of epithelial sheets during wound healing, in vivo.
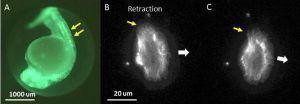
Panel A. A fluorescence image of A zebrafish embryo expressing GFP-Utrophin that labels the actin cytoskeleton (green) in all tissues, including epithelial cells along the back of the embryo (double yellow arrows). Panels B and C. A single, transgenic zebrafish epithelial cell expressing GFP-Utrophin, which labels all of the cytoskeletal actin, seen here as a diffuse, filamentous structure. This cell is moving in the direction indicated (large, white arrow) and retracting on its left edge (yellow arrow) causing it to turn to the right. The time interval between images is ~ 2 minutes.
How is the movement of epithelial cell sheets coordinated during the wound healing response?
One of the reasons that fish keratocytes move so rapidly is because they are involved in wound healing. The initial stages of wound healing can be observed in vitro, within 24 hours, as epithelial cell sheets migrate out from the edge of the scale. After 48 hours, individual keratocytes begin to disperse from the edge of the cell sheet and it begins to break up. We have observed calcium transients within sheets of cells that appear to travel from cell to cell. When calcium transients are inhibited parts of the sheet begin to pull away from each other and holes begin to appear as the integrity of the epithelial cell sheet is lost. This finding together with previous studies of wound healing suggest that calcium signaling is important for coordinating the movement of individual cells within a sheet such that they can move forward as a single unit. If so then we would expect calcium transients to propagate from one cell to the other, rather than occurring randomly within the cell sheet.
Calcium signaling between cells in an epithelial cell sheet
The movie shows a pseudo-colored image of a sheet of fish epithelial keratocytes loaded with a fluorescent calcium indicator, which fluoresces more brightly (yellow and red) when there is an increase in [Ca2+]i. Calcium transients can be seen apparently passing between cells in a sheet. This movie sequence represents 1.5 minutes of real time, with each image acquired every 0.5 seconds.
One of the goals of this work is to determine whether calcium signaling is occurring randomly or is propagating from one cell to the other. To test this possibility, we are using a photoactivatable ionophore to induce a calcium transient in a single cell and then observing whether calcium transients propagate outward from this cell. To determine whether stretch-activated channels (SACs) are involved in the propagation of transients, we are using inhibitors to block gap junctions or SACs to see whether either of these treatments prevent the propagation of transients between cells. Understanding the factors that control the integrity of cell sheets may have clinical implications regarding the development of treatments to promote wound healing or to inhibit cell scattering during cancer metastasis.